Research Highlights on Journal Covers




Research Overview
Biomolecular motors and their associated proteins play crucial roles in a wide range of functions in living organisms. Among them, microtubule is the most rigid protein in the eukaryotic cytoskeleton, which serves as one of the major structural components in cells. Microtubules, in cooperation with their associated motor proteins, such as kinesin, dynein form the most prominent biomolecular motor system in cells. Roles of microtubules and their associated motor proteins are critical for ensuring life and dynamics of living beings. My research activities are aimed at exploring the diverse roles of biomolecular motors and their associated proteins in cells and unravelling the underlying mechanisms. I also have deep fascination about possible applications of those natural machineries in fabrication of artificial micro/nano devices, molecular robots, next generation smart machineries for various real life applications.
Development of a minimal artificial brain by the power of chemistry
I have been working to construct a molecular system with complex functions by mounting various molecules with different functions such as sensors, processors, and actuators. The goal is to develop artificial cells and encapsulate the molecular components inside the cells so that the cells may work as independent autonomous entity like the brain in living organisms. A novel methodology is adopted based on the integration of all the functions through chemical reactions between molecules in a bottom up manner. Particularly, I focus on utilization of biomolecular motor proteins to build up an actuation system for the minimal artificial brain that will permit communication among the groups of artificial cells as a neural network. See for details.

Artificial cells for understanding the mechanism of life
Lipid vesicles offer a versatile platform in construction of artificial cells. Recent progress in minimal cell research has been greatly benefitted by the technological advancements of lipid-based artificial cells. Encapsulation of biologically active components, for example, proteins, genes, enzymes, or other cellular structures, in artificial cells offer great potentials in understanding the origin of life. The artificial cells are promising for many real-life applications, such as drug delivery, molecular robotics, and so on. In my research, I utilize such cell-free expression system to understand the functions of cytoskeletal proteins and their organization in a life-like environment.


Mechanobiology of cytoskeletons and its connection to neurological diseases
Microtubule, the most rigid component of cytoskeleton and nerve cells, not only provides structural supports to cells but also plays pivotal roles in cellular mechano-transduction, according to the prevailing view. Mechanical forces are major regulators of cellular functions and microtubules, together with associated motors proteins, are believed to be among the major players in mechano-regulation of cellular functions. My research works are dedicated to understand the role of microtubules as a mechano-transducer, which motivated me to study the impact of mechanical forces on the structure and biochemical functions of microtubules and their associated proteins.


Deformation of microtubules under compressive (left) and tensile (right) mechanical force.
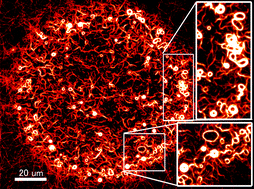
Active self-assembly of biomolecular motors
Biomolecular motors are promising candidates for fabricating artificial molecular devices. however, their nanoscale dimension has been an impediment, which can be overcome through hierarchical structural integration. Active self-assembly of biomolecular motors has been an efficient route to scale up the molecular components into micro-meso scale organized structures. So far, active self-assembly has been successfully employed to synthesize large scale bundles, ring-shaped, and giant network structures from biomolecular motor proteins. All the self-assembled structures are potential candidates in fabricating molecular machines/devices for nanotechnological applications.
Fabrication of molecular robots through multidisciplinary approaches
Biomolecular motors can generate force on their own and perform mechanical work by consuming chemical energy. These features have rendered the group of cytoskeletal proteins as an ideal candidate for fabricating molecular robots, through fusion of DNA nanotechnology. The biomolecular motors and DNA have been employed as the actuator and processor of the robots. Inclusion of photo-responsive chemicals has facilitated the robots with sensing ability. Such multidisciplinary approaches have led to what we envision as the future player in the world of artificial intelligence in the molecular world. See for details.

Regulation of the activity of biomolecular motors
With the advent of engineering methodologies for finding real life applications of biomolecular motors, it has become inevitable to achieve control over the functions of biomolecular based molecular devices. Valiant efforts have been devoted in quest of technology that could permit controlling the functions of biomolecular motors in a used-defined manner. To date, I have discovered that the deep-sea the osmolyte trimethylamine N-oxide (TMAO) offers a convenient means to control the activity of biomolecular motors in a robust, reversible and repeatable manner. This finding raises critical questions on the role of natural osmolytes in connection with the stability, activity of motor proteins and various diseases in living organisms, which are of great physiological significance.

Improving thermal stability of motor proteins
Biomolecular motor proteins and their associated cytoskeletal proteins work well in living organisms even at temperature as high as ~40 C. However, the reconstructed proteins readily loss their activity at such high temperatures in the artificial environment or when subjected to prolonged operation even at at room temperature. Such functional impairment of the reconstructed motor proteins has been a serious drawback in the applications of biomolecular motors in various field. One of my research interests has been directed to improve the thermal stability of reconstructed biomolecular motors and their associated proteins. I have adopted point mutation as a strategy to improve the thermal stability of the motor protein kinesin. This approach involves substitution of one/more amino acid residue(s) in the amino acid sequence of the kinesin with suitable amino acids which results in energetically favorable state for the mutant kinesin at temperatures higher than the ambient temperature. Synthesis of heat-tolerant motor protein is expected to benefit nanotechnology, molecular robotics and material science.
Research Video Gallery











